Gary Anderson created a recycling symbol in 1970, and though the design evolved in the first few years, the following image is now known as the universal recycling symbol.¹
The symbol is recognized as filled in (as above), outlined, colored, in and out of a circle, and with a simple or complex bend. All variations represent recycling, but some variations carry more meaning than others, such as the resin identification codes examined in Comparing Plastics.
The three arrows broadly represent the three tenets: recycle, reduce, reuse. They form a continuous circle (more accurately, triangle) representing the ideal of sustainability.
Project or print an example of the universal recycling symbol for your class and ask them to interpret it using any materials, colors, or textures that inspire them. Their symbol should follow the broad guidelines of the original, but they should feel free to embrace their creativity.
Students should share their interpretations with the class. Are there certain themes or motifs that repeat? Are all of the symbols recognizable? Ask students (or a sample of students) to explain their artistic choices.
Different parts of the world and different industries use slightly different symbols to communicate that an item is able to be recycled or to communicate the properties of that item. Some of those different examples are included below. Project the different symbols or print the attached sheet and ask students to compare and contrast the symbols based on their qualities.

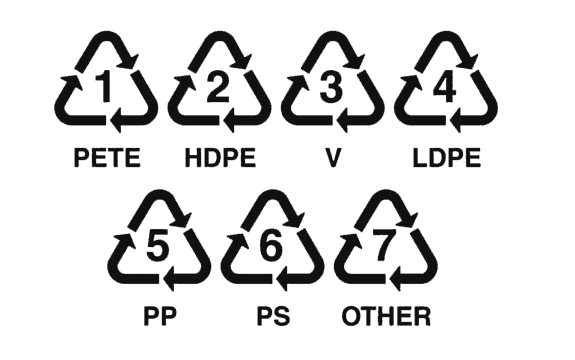


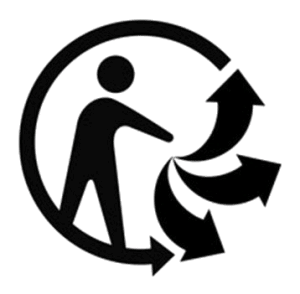
Students should notice that all of the symbols use arrows, in some way or another. Some of the symbols on this page use the color green. All of them are very simple designs. Which communicates more information? Do they communicate it clearly? Which have the students seen before? Are any surprising? Do students have a favorite? Why is it inspiring or noteworthy?
You may challenge older students to find more symbols using the Internet or reference materials or lead students through a quick search for more regional or country-specific symbols or labels. Extend the questions posed above to any symbols or labels you find.
Assign one of the symbols above or another symbol relating to resources and recycling to individual students or groups of students. Ask students to research the following questions using external resources. Students should be prepared to present their findings to the class with a visual representation of their symbol, either printed or digitally. Students should cite their sources appropriately.
After hearing from their classmates about the different signs and signifiers used in recycling symbols around the world, ask students to redesign the recycling symbol. Allow students to draw inspiration from what they have learned. Their ideas can include similarities from any of the existing symbols in use but should show a level of artistic or symbolic interpretation.
Students should then compose a brief write-up containing the elements they selected for their symbol and the meaning thereof. This write up should also reference any symbols from which they drew inspiration. Their self-analysis should contain references to form, color, texture, and symbolic function if appropriate.
TEKS
ART.K.2A, ART.K.2B, ART.1.2A, ART.1.2B, ART.2.2A, ART.2.2B, ART.3.2A, ART.3.2B, ART.3.2A, ART.3.2B, ART.4.2A, ART.4.2B, ART.5.2A, ART.5.2B, ART.1.1A, ART.2.1A, ART.4.1A, ART.5.1A
ELA.3.25B, ELA.4.25B, ELA.5.25B, ELA.3.26A.i, ELA.4.24A.i, ELA.4.24A.i, ELA.3.28A, ELA.4.26A, ELA.5.26C
ART.4.4B, ART.5.4B, ART.4.2B, ART.5.2B

We'd love to help answer any questions and help you get started! Drop us a line and we'll get back to you as soon as we can.
Watt Watchers of Texas
204 E. Dean Keeton Street, Austin, Texas 78712
contact@watt-watchers.com
Nos encantaría contestarle cualquier pregunta que tenga y ayudarle empezar! Envíenos un mensaje y nos pondremos en contacto con usted lo antes posible.
Watt Watchers de Texas
204 E. Dean Keeton Street, Austin, Texas 78712
contact@watt-watchers.com